Taking it to the Next Level with Acid Dyes
Premetallized acid dye make a lot of deeper or earthier colors possible, and are the most permanent of all the acid dyes, but they can be a little tricky to get an even shade (called leveling) with because they are so eager to fix to the fabric. To help with this we recommend using Ammonium Sulfate. But how do you use it? Does it really make a difference? How much do you need? We did a series of tests with two of our more advanced colors, 438 Olive Brown and 431 Lilac, to help demonstrate.
Before we get going here is a quick re-cap of just what Premetallized Acid Dyes are:
Premetallized acid dyes are even larger acid dye molecules complexed with an ion of a metal, usually chromium or cobalt in a trivalent form, which is relatively safe when handled properly, and present in such minute quantities that by the time it is diluted with water, it more than meets EPA requirements for disposal. These dyes are very wash fast and do not migrate once they fix to the fiber, so you must be extra extra careful to apply them evenly. Proper agitation of the dyebath and the addition of Ammonium Sulfate as a leveling agent, just like with the Milling acid dyes, should solve this. These are the most wash fast and light fast of all of the classes of acid dyes.
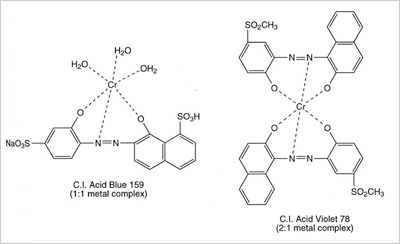
Premetallized acid dyes fall into two classes, 1:1 metal-complexes, in which one dye molecule is complexed with one metal atom and the more modern 1:2 metal complexes, in which one metal atom is complexed with two dye molecules. The dye molecule is typically a monoazo structure containing additional groups such as hydroxyl, carboxyl or amino groups, which are capable of forming strong co-ordination complexes with transition metal ions, typically chromium, or cobalt.
Using Amonium Sulfate
Ammonium Sulfate is a leveling agent, which means it slows the absorption of the dye into the fiber. It also causes to dyebath to become acidic very gradually, so the dye fixes over time rather than all at once. By slowing the rate at which the fiber absorbs the dye, and the rate at which the dye fixes, the dye molecules have a much greater chance to evenly penetrate the fiber giving us a more even color over all. It also happens to be a great fertilizer.
We did five tests to demonstrate the effect Ammonium Sulfate can have on your results. Because of our sample sizes we used solutions of Citric Acid and Ammonium Sulfate created with 1 tbls dry powder to 16 oz of water.
1st Test: Control—Used 2 tsp Citric Acid solution—mottled
2nd Test: Just Ammonium Sulfate—2 tsp of solution—did not exhaust, all color did not strike
3rd Test: 4 tsp of Ammonium Sulfate solution—some did, not all
4th Test: 2 tsp each Citric Acid and Ammonium Sulfate solutions added at the same time—mottled, struck too fast as with 1st Test.
5th Test: 2 tsp Ammonium Sulfate solution used at start of bath, 2 tsp citric acid solution added at the end of the bath—perfect! Even. Exhausted.
The best results are to be had when you add the Ammonium Sulfate at the beginning of the dye bath, and then once the water comes up to temperature and has simmered for 30 minutes, adding the Citric Acid. This will cause the last of the dye to exhaust and give you the full depth of shade and the most even color. It is also important to stir very frequently and bring the temperature up slowly.
Here you can see the dramatic difference more clearly between just using Citric Acid and using the Ammonium Sulfate with Citric add at the end:
So how much Ammonium Sulfate and Citric Acid should you use?

The rich colors to be had from Premetallized Acid Dyes are definitely worth the small bit of extra care they need. Using a leveling agent will not only give you more even dye jobs, but it will make repeating dye results much easier.
Happy Dyeing!
Items used in this article:
Wool Yarn
Other Related Articles: